BMC’s Clinical Trial Office (CTO) serves as a central resource for principal investigators, study staff, and departments involved in clinical research, and for sponsors seeking to conduct clinical trials at BMC. Our CTO pre-award and post-award teams support and advance BMC’s mission by providing leadership and expertise in Research, Finance, and Administration.
September 2024
CTO Announces Change to Speed Up Clinical Trial Agreement Execution
Currently, the Informed Consent Form (ICF) is 1 of 4 required documents (ICF, Clinical Trial Agreement, study budget or funding sheet, and comprehensive protocol) uploaded by the study team into Velos to initiate CTO’s clinical trial pre-award support services.
To address 2-3 week sponsor delays in supplying the study team a copy of the ICF, CTO is implementing a change to expedite the pre-award process. Going forward, the ICF draft will no longer be required for CTO to start the review process. Instead, study teams should upload the ICF to Velos as soon as possible, the CTO must have a copy of the ICF before the first round of external negotiations- a delayed ICF upload will delay negotiations.
This adjustment in practice aims to remain competitive in our study start-up process by reducing days to execute the Clinical Trial Agreement.
New Clinical Trials Budgeting & Billing Working Group: Join the Conversation
We are excited to announce the formation of a new working group dedicated to enhancing our approach to budgeting and billing within clinical trials. This group will serve as a collaborative platform for team members to share insights, discuss challenges, and develop strategies to optimize our financial processes. Our primary focus will be on ensuring that our budgeting practices are both efficient and compliant with the latest guidelines. By coming together, we aim to streamline our procedures, ensure comprehensive coverage of trial-related expenses, and ultimately contribute to the financial success of our clinical trials.
If you are interested in joining, please email cto@bmc.org!
First meeting Date: 10/2, 1-2 PM
Agenda
- Review of New Changes in Budgeting
- Clinical Trial Start-Up Costs:
- Evaluation of current start-up cost grouping methods.
- Changes to CCRA start-up costs including the addition of coverage analysis.
- Exploration of potential improvements to streamline and standardize these costs across trials.
- Coverage for Fees on ClinCard:
- Examination of the new policies regarding fee coverage for the use of ClinCards.
- Discussion on how we’ll incorporate these fees into trial budgets.
- Topics for the next meeting in late September
ClinCard Cost Coverage for Industry Sponsors
The ClinCard program began as a pilot approximately 7 years ago and has since become our primary method for participant remunerations. To facilitate this transition, the hospital has been covering the associated costs, including monthly administrative fees, card issuance, and transaction fees. However, as our use of ClinCard has grown, so have the costs. We now distribute around 5,000 cards and process over 12,000 payments annually. Each card costs $4, and each payment incurs a fee of $1.15. This represents a substantial expense for the hospital.
To ensure the sustainability of this valuable service, we will now be asking industry sponsors to cover the costs associated to their studies. Starting on October 1st 2024, we will request that industry sponsors cover the ClinCard-related costs specific to their studies when negotiating budgets with them. This change will allow us to continue providing this convenient payment method without impacting the hospital’s financial resources.
Which studies will the cost recovery impact?
- Industry studies that are currently in negotiations or in a pre-award state starting October 1st. CTO will negotiate budgets to ensure this cost is covered.
- Existing studies will not be impacted.
What are the new costs?
- $4 fee for each physical card.
- $1.15 transaction fee for each successful payment.
Table 1 shows how new fees structure will be applied to new studies
August 2024
BU CTSI Knowledge Sharing Platform
Johanna Chesley, a Senior Director of the Center for Clinical Research Advancement, and Cindy Hu, a CTO intern, recently participated in a significant knowledge-sharing event organized by the BU CTSI. Focused on research workforce diversity, the CTSI Pilot: Knowledge Sharing Platform brought together key CTSI partners from Boston academic centers, community organizations, and government representatives. During the event, key findings from a scoping literature review were shared, and attendees discussed not only mechanisms to diversify the research workforce but also innovative strategies to engage the next generation of research staff. Read more
July 2024
Clinical Research Billing and Finance, Part IV
Sponsored by the CTO, this session of the Clinical Research Billing and Finance series returns first to the topic of internal budgeting, for a deep dive into projecting effort and its costs, and then reviews effort reporting and salary allocation. Presenters include subject matter experts from the CTO, the Clinical Research Network, and Sponsored Programs Finance (SPF).
Please note: this presentation does not include training in effort certification. That training is found in the SPF training library located below the education calendar for August 7 from 2-3:30pm: https://www.bmc.org/research-operations/research-education/calendar
Objectives
- Understand how to project time and financial costs of CR study activities
- Recognize the risks of non-compliance in effort reporting
- Identify the component parts of effort reporting and salary allocation, and
- Gain confidence in completing effort reporting accurately.
Pre-registration is not necessary.
A recording with slides will become available within 10 days after each CRBF session. All recordings are available in the web library located beneath the education calendar.
Investigators, department administrators, and CR study staff are all invited to attend. If you have not already received the meeting request, please contact Rhonda Champagnie, Executive Assistant, Office of the Chief Scientific Officer, and ask to be added to the distribution group associated with your BMC/BUMC role.
Ask the Experts
Dear clinical research colleagues:
We invite you to the "Ask the Experts" office hour, where you can consult members of our CTO team on any questions you have or challenges you face in following the clinical research billing and finance (CRBF) process, including use of its related systems: Velos, Epic, and/or ClinCard. Your participation will help the CTO develop training and job aids.
In particular, it is crucial for us to understand the challenges the study team faces in updating patient status in Velos. We aim to learn about any issues you may be experiencing when updating statuses so that we can provide appropriate support. But all topics are welcome!
June 2024
The Clinical Research Unit (CRU)


The Clinical Research Unit (CRU) is Boston Medical Center’s newly established, state-of-the-art 3,000 sq. ft. clinical research facility, located on the 6th floor of the Yawkey building. The CRU is dedicated to spearheading medical advancements and centered around the patient experience to deliver the highest level of safety, compliance, and service excellence.
Since 2021, the CRU has worked in close collaboration with various hospital leaders, physician representatives, community members, CTSI and GCRU, ancillary service departments, and industry partners to meet the needs of BMC/BU’s expanding research portfolio.

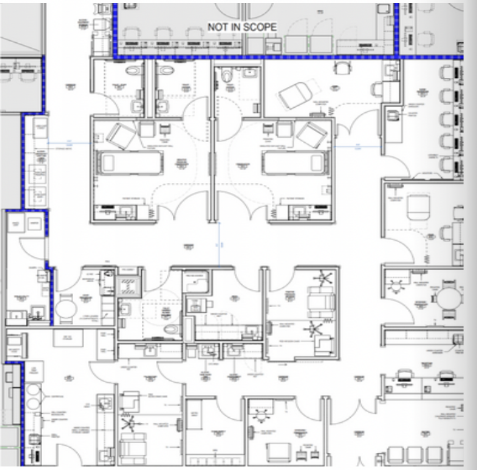
Aside from being staffed by a dedicated team of hematology/oncology nurses, clinical coordinators, and Medical Director, the CRU also houses a comprehensive array of pediatric and adult facilities: infusion and clinical exam rooms, phlebotomy space, The Andry Mini Lab, medication and nutrition stations, code carts, and overnight infusion/observation rooms.
Reflecting BMCs culture of compassion and diversity, the CRU is committed to advancing science inclusively as we strive toward health equity. In line with this commitment, the CRU aims to not only diversify the workforce, ranging from faculty to nurses, but also cultivate the development of future research staff through the application of programmatic approaches to mentorship and training. Additionally, the CRU seeks to strengthen trust and partnerships with the communities we serve.
With sincere appreciation we acknowledge Dr. Ravin Davidoff and Terri Newson for their advocacy to develop the CRU. And to Alex Walker for supporting the project management unit build. Projected opening is Nov/Dec 2024.
The Clinical Research Unit
Expand research portfolio - Advance science inclusively - Diversify workforce - Enhance partnerships
Internal Cost Budgeting presentation, Thursday, June 20, 2-3:30 pm
Part III of the Clinical Research Billing and Finance (CRBF) series, sponsored by the Clinical Trial Office, is devoted to Internal Budgeting. Accurate, thorough budgeting of project costs is crucial for protecting institutional resources, ensuring transparency, and staying compliant with regulations.
In this session, you'll learn about the roles, responsibilities, and best practices for budgeting clinical research studies. Through interactive examples and open discussion, you'll gain the knowledge to confidently project costs for all phases of a study – from start-up to close-out.
Don't miss this opportunity to:
- Understand why thorough budgeting is essential for safeguarding resources and assuring compliance
- Clarify the budgeting roles of departments and oversight groups, and
- Build expertise in comprehensively forecasting study expenses.
This is a valuable chance to strengthen your financial management skills for clinical research.
We are Hiring!
We're Excited to Hire a Senior Clinical Research Financial Analyst!
Calling all finance wizards with at least 5 years of professional experience in research administration or financial analysis/accounting! The ideal candidate has significant experience in research finance duties, eg, clinical trials budgeting and accounting. We especially appreciate candidates with the BMC departmental perspective.
We have an amazing opportunity for you to join our dynamic team and contribute to a wide range of groundbreaking biomedical research. Get ready to innovate, whether it’s developing budgets that fuel cutting-edge clinical trials, analyzing financial data, providing game-changing insights, monitoring projects, ensuing funds are optimized, and collaborating with brilliant research minds.
What You'll Bring: A bachelor's degree (extra points for finance-related education!), comfort with MS Office, the ability to master new systems, a mind for analytics, a keen eye for details, and top-notch communication abilities.
Don't miss this chance to be part of the action! Submit your resume and get ready to make an impact. Apply here
May 2024
Two-Year Series on Clinical Research Billing and Finance
The Clinical Trial Office is pleased to announce the commencement of a two-year series on clinical research billing and finance. The series will largely follow the chronological process after introductions to research billing compliance and to the process itself.
CTO Senior Manager Mike Porreca and Research Operations’ education liaison Kaye Mottola are leading program development. Ryan Schroeder and Quinneil Simmons of the Clinical Research Network will lead the internal cost budgeting session this summer.
Research Operations will send out meeting requests as the courses are scheduled. Please see the research education page for dates and times.
Clinical Research Billing and Finance series:
- The Basics of Research Billing Compliance
- Introduction to the Research Billing and Finance Process
- Fundamentals of Internal Cost Budgeting of Clinical Research
- Focus on Effort Cost Budgeting of Clinical Research
New Role for CTO Team Member
We have exciting updates about new responsibilities within CTO.
We are pleased to announce that Sandy Lok has taken on the role of CTO's Contract Specialist. Sandy's primary responsibility in this position is to review clinical trial and confidentiality disclosure agreements. Her extensive background as a senior clinical trial financial analyst and her deep knowledge and expertise will prove invaluable as she handles the critical task of contract review and analysis.
Ask the Experts
We continue to conduct our monthly "Ask the Experts" sessions, where we discuss best practices for working in Velos and ClinCard, and review critical issues for research billing. We encourage everyone to attend these informative sessions to learn and clarify any questions you may have.
April 2024
Research Billing Updates
The research billing review process at BMC depends significantly on study teams keeping diligent records. Accurately tracking patients' participation in Velos is critical to prevent billing errors for research services.
As part of our team's ongoing efforts to maintain accurate and current clinical trial records in the Velos system, Clinical Trials Office has identified a critical need for updating patient statuses. Our data analyst, Tran Trinh will be reaching out to each department with the list of patient IDs and the new guide on how to update the patient's existing status in Velos.
Ask the Experts
We'd also like to invite you to join CTO's Office Hours, "Ask The Experts" session. Join us monthly in the Q&A session to learn tips and tricks for navigating the ClinCard, VelosCT, and Research Billing Review process.
You can join the Zoom Q&A anytime during the one-hour meeting and ask us questions relating to your studies. Feel free to submit any inquiries you may have in this form: https://bmc.tfaforms.net/261
We will provide you with meeting logistics shortly. Stay tuned!
March 2024
Harley Jacobsen Clinical Trial Participant Income Exemption Act

On January 29th, 2024, U.S. Reps. Mike Kelly (R-PA) and Chrissy Houlahan (D-PA) introduced the Harley-Jacobsen Clinical Trial Participant Income Exemption Act, proposed legislation aiming to exempt all remuneration payments received by participants in clinical trials from being counted towards their gross income.
Current Limitations:
Under current law, remuneration payments are considered taxable income as they are not categorized as reimbursements, creating a financial burden for participants in lower income brackets.
Benefits of the Harley Jacobsen Clinical Trial Participant Income Exemption Act:
- Increased Diversity in Trials:
- The Act aims to diversify patient populations in clinical trials, aligning with NIH and FDA goals. This improves drug development effectiveness for the public.
- Greater Access to Therapies:
- It provides financial support to marginalized groups, ensuring broader access to experimental therapies, including those with disabilities, minorities, and low-income individuals.
- Streamlined Reporting:
- The Act eliminates reporting requirements for both participants and payers, protecting those reliant on social welfare programs from exceeding income limits.
Read more:
- Kelly, Houlahan introduce "Harley Jacobsen Clinical Trial Participant Income Exemption Act" | Congressman Mike Kelly (house.gov)
- H.R.7090 - 118th Congress (2023-2024): To amend the Internal Revenue Code of 1986 to exclude from gross income certain compensation to clinical trial participants. | Congress.gov | Library of Congress
Ask the Experts
On March 12th, CTO will be hosting “Ask the Experts”, see below for more details
- Zoom: https://bostonmedicalcenter.zoom.us/j/94056779139
- Submit questions: https://bmc.tfaforms.net/261
January/February 2024
Research billing updates
The Clinical Trial Office would like to welcome and express gratitude for Natalia Kats who transitioned on 12/12/23 as the CTO African Bridge Network Fellow into a temporary coverage role in research billing compliance through 2/12/24. Also for cross-training purposes we will be training two Revenue Cycle employees to serve as backup on the research billing workflow.
In order to help us protect the participants and institution from misbilling, we would like to request that study teams respond rapidly to Natalia and Revenue Cycle’s charge routing inquiries.
Several study teams have responded rapidly to Natalia’s inquires and we would like to acknowledge their support:
- InSTI Weight Gain
- RABIT
- Understanding the Long-term Impact of COVID-19 in Adults studies
We are currently reviewing and optimizing the research billing process in hopes of identifying several manual and system related improvement.
Ask the experts
Do you have questions about ClinCard, VelosCT, or the Research Billing Review process? Do you want to learn some tips and tricks for navigating these systems? Are you curious about your role in these workflows overall?
If yes, then please join us in our Ask the Experts Q&A session, where CTO will share tips and tricks, and answer any questions you may have about ClinCard, VelosCT, or research billing.
The next session will be on Tuesday, March 12, 2024 from 2:00pm-3:00pm, with future sessions planned each new month. If you are interested in joining one or multiple meetings, please sign up here. Feel free to also submit any inquiries you may have in the form and CTO will address them during the session. Attendees will receive an Outlook invite with a zoom link after signing up.
Current agenda for the Ask the Experts Q&A session:
- Navigating the new CTO webpages - ClinCard & VelosCT
- ClinCard & VelosCT workflow updates, tips & guides
- Research billing reminders
- Questions submitted for CTO
If you have general questions or would like more information about our Ask the Experts session, please contact
CTO@bmc.org.
December 2023
African Bridge Network: 2023 Fellows at BMC
We, the 2023 ABN fellows, are absolutely thrilled to introduce ourselves to the BMC community. A diverse group of four individuals with unique backgrounds, yet we share a common goal: establishing a career in the US healthcare field.
Each one of us brings distinct expertise to BMC. Gina, with a background in clinical laboratory and research, is leveraging her proficiency within the CRN team. Natalia, with her deep expertise in marketing and finance, is bringing her skills to the CTO team. Raghda is expanding her skills in IT and project management at the Clinical Data Warehouse, Research. Finally, Saber, experienced in community-based development and international medical research, enriches the Massachusetts Community Engagement Alliance program with his valuable talents.
Thanks to African Bridge Network (ABN) and their robust immigrant professional fellowships, all of us were able to complete the Research Administration (RA) course at Emmanuel College in addition to a soft skills training prior to joining BMC. This training made us more familiar with the American market and the terminology used in scientific centers. This fellowship has been an incredible opportunity as it offers multiple tracks (Finance, RA and Project Management), allowing for a good fit between position and intern.
We are fortunate to have matched with BMC, a unique organization with outstanding services. BMC has welcomed us in every possible way, providing us with useful insights, a profound understanding of how research operates at BMC and hands-on experience.
In less than two months, we have found ourselves deeply committed to BMC's mission. We challenge ourselves daily and experience significant professional and personal growth. We feel valued here at BMC.
We want to take this opportunity to express our heartfelt thanks to every member of our teams and every colleague at BMC. Your support has made this journey both exciting and fruitful.
As we continue our journey with BMC, we are eager to contribute our skills and knowledge to further the organization's mission. We are seeking full-time employment opportunities at BMC. Please, contact us if your department has open positions that would benefit from new perspectives and hard-working, and passionate individuals who care about the advancement of science for all.
King regards,
Gina, Natalia, Raghda, Saber

Please contact Johanna Chesley (email: Johanna.Chesley@bmc.org) if you are interested in hosting future ABN fellows in research administration.
Internships commence in September, 2024, and run for 12 weeks.
November 2023
The Clinical Trial Office (CTO) would like to highlight the following points to the community.
Confidential Disclosure & Nondisclosure Agreements (CDAs & NDAs)
The CTO has a centralized review process for all Confidential Disclosure Agreements (CDAs) and Nondisclosure Agreements (NDAs) relating to potential research projects. Study teams should submit CDAs and NDAs to the CTO via our CDA/NDA intake form.
For questions regarding CDAs/NDAs, please contact Sandy.Lok@bmc.org.
VelosCT Data Entry
BMC’s research billing review process is dependent on study teams entering data in VelosCT in an accurate and timely manner. Study teams must enroll participants within 24 hours of the participant’s first research visit and update all other calendar visits/events within 24-48 hours of the encounter. Study teams must also update the participants’ statuses in VelosCT when they have screen-failed or completed the study.
This is crucial to ensure compliant billing to-third party payers, prevent incorrect billing of research services, and maintain participants’ trust and confidentiality.
For questions about the research billing review process, please contact your department assigned Clinical Trial Financial Analyst (CTFA) or email CTO@bmc.org.
Remote Monitoring Workflow
BMC allows and supports remote monitoring for research studies via Chartlink. To ensure that studies and monitors are set up properly, study teams must complete these steps:
- Chartlink study setup – Work with the CTO to obtain a signed Memorandum of Agreement (MOA) with the sponsor or Clinical Research Organization (CRO).
- Monitor setup – After the MOA is signed, have each monitor sign a Confidentiality Disclosure Agreement (CDA) – Exhibit in MOA. The CDA must include the name of the monitor and the study or studies to which access is required.
- Participant Linking - After research studies and monitors are set up in Chartlink, link participants to Chartlink for review.
For questions regarding the remote monitoring process, please contact your department-assigned Clinical Trial Financial Analyst or email CTO@bmc.org.
We hope that these points will help study teams manage their research projects more smoothly.
October 2023
Investigational Pharmacy Service Budget Updates
IPS is a service center located in the Department of Pharmacy at BMC. IPS is designated to handle, control and store all medications, including Investigational Products (IP), which are part of a BMC or BUMC clinical trial. IPS is funded through user fees charged to investigators and sponsors. These fees are based on costs to deliver the IPS services
It is the goal of the BMC IPS to recover the full costs of each individual sponsored project, where permitted by the established policies of the funding agency, and with a secondary target to break even the cost of operating the IPS department.
Key updates:
- New billing hourly rate:
- Federal billed at $90 per hour
- Industry billed at $150 per hour
- Rate Change
- Increases the fee for sections reliant on that rate calculation (i.e. initiation, close-out, & maintenance)
- Archive fee increased from $100 to $125 for both industry and federal studies
- Monitoring fees were increased from $125 remote and $50 on-site to:
- $150 remote (both federal and industry)
- $75 on-site (both federal and industry)
- Protocol Amendment Fees:
- Federal $270 per amendment (3 hrs/amend x $90/hr)
- Industry $450 per amendment (3 hrs/amend x$150/hr)
- Maintenance fees
- Begin once the investigational product is received from the sponsor and continue to accrue until the study drug is returned or destroyed
- Annual Inflation Adjustment
- A three percent increase in the hourly rate calculation will be applied annually starting on 01-Oct-2024.
- This applies to new budget negotiations only
- Piloting a change to monthly invoicing (currently quarterly) starting Oct 1st.
Background information:
The consumer price index (CPI) increased 21.06% from June-2018 to May-2023. Our last hourly rate update was on 6/1/2018:
- Federal billed at $75 per hour
- Industry billed at $125 per hour
The new billing hourly rate (rounded to the nearest whole number) based on the US Bureaus of Labor Statistics CPI calculator:
- Federal billed at $90 per hour
- Industry billed at $150 per hour
The data was based on the federal site:
https://www.bls.gov/data/inflation_calculator.htm
September 2023
Clinical Research Space Design is really happening!
The JCRU unit layout has been finalized. We are now moving forward with 3-D design room-by-room with BMC subject matter experts, BMC Expansion 2.0 project management, and Architectural Firm. In addition to room design we are beginning to think about JCRU charter, workflows, staffing model, and SOPs with multiple partners across BMC and BU.
Check back for updates.
August 2023
Research Operations is excited to announce re-branding of The Clinical Trial Office teams into The Center for Clinical Research Advancement.
June 2023
Upcoming Workshop: The Ecosystem of Health Equity Measures
June 21, 2023 | 8:30 a.m. to 4:30 p.m. ET

On June 21, the National Academies’ Roundtable on Population Health Improvement will host a public workshop to examine the state, use of, research on, and effects of health equity metrics at every level of the health ecosystem. Invited presenters will discuss the health equity metrics currently used in the health (clinical) sector and beyond, the methods for evaluating metrics’ efficacy, and the implementation strategies to use metrics for accountability and transparency. Speakers will also review the state of the science and practice for specific social determinants of health relevant to health equity (e.g., community power, civic engagement). The session will build on earlier exploration of how health equity is measured and the factors that shape it in “Metrics that Matter for Population Health Action.”
The event will be held in-person and accessible via live webcast. Find more information on the event page.
May 2023
Checkout the recent CR Times spotlight written in collaboration with the BMC Clinical Trial Office and Clinical Research Network Participation Reimbursement working group. Click here to read.
Learn More--CR Times Article covers:
1. Summary of the ClinCard Program at BMC
2. Review ClinCard Satisfaction Survey Results
3. Gain access to ClinCard training opportunities
4. Access FAQs for study teams
5. ClinCard Conclusion and Reminders
6. Review alternative or supplement reimbursement options
SEEKING DEPARTMENTAL REPRESENATION TO JOIN PARTICIPANT REIMBURSEMENT WORKING GROUP. If interested, please contact: |
April 2023
ClinCard Customer Service Satisfaction Survey Results
The Clinical Trial Office and Clinical Research Network requested your input on ClinCard satisfaction. 90 researchers responded. Read More to view the results and tune in next month for a survey response summary from The Participant Reimbursement Working Group.
Looking for departmental Participant Working Group representatives.
Please, email: CTO@BMC.org for more information.
March 2023
Reminder to study teams
Reminder to study teams to update participant statuses in VelosCT. After enrolling the participants, study teams must make sure to update the status again when the participants complete, screen fail, or withdraw from studies. When participants are left on studies in VelosCT and Epic, this adversely affects CTO’s and Revenue Integrity’s billing workflows:
- Claims that are not research-related will be unnecessarily delayed in billing out to third-party payers, running the risk of missing the timely filing limit.
- There is a possibility to misbill charges, which can lead to mistrust with our patients and potential confidentiality breech.
- Participants will be left incorrectly enrolled in studies, which can lead to audit risks/findings
If study teams are unsure how to update participants statuses, please contact your department-assigned Clinical Trial Financial Analyst or email CTO@bmc.org. You can also review the VelosCT training materials on the CTO website (Systems > VelosCT).
 en
en 

 Français
Français Deutsch
Deutsch Italiano
Italiano Español
Español Tiếng Việt
Tiếng Việt Kreyol ayisyen
Kreyol ayisyen