Overview
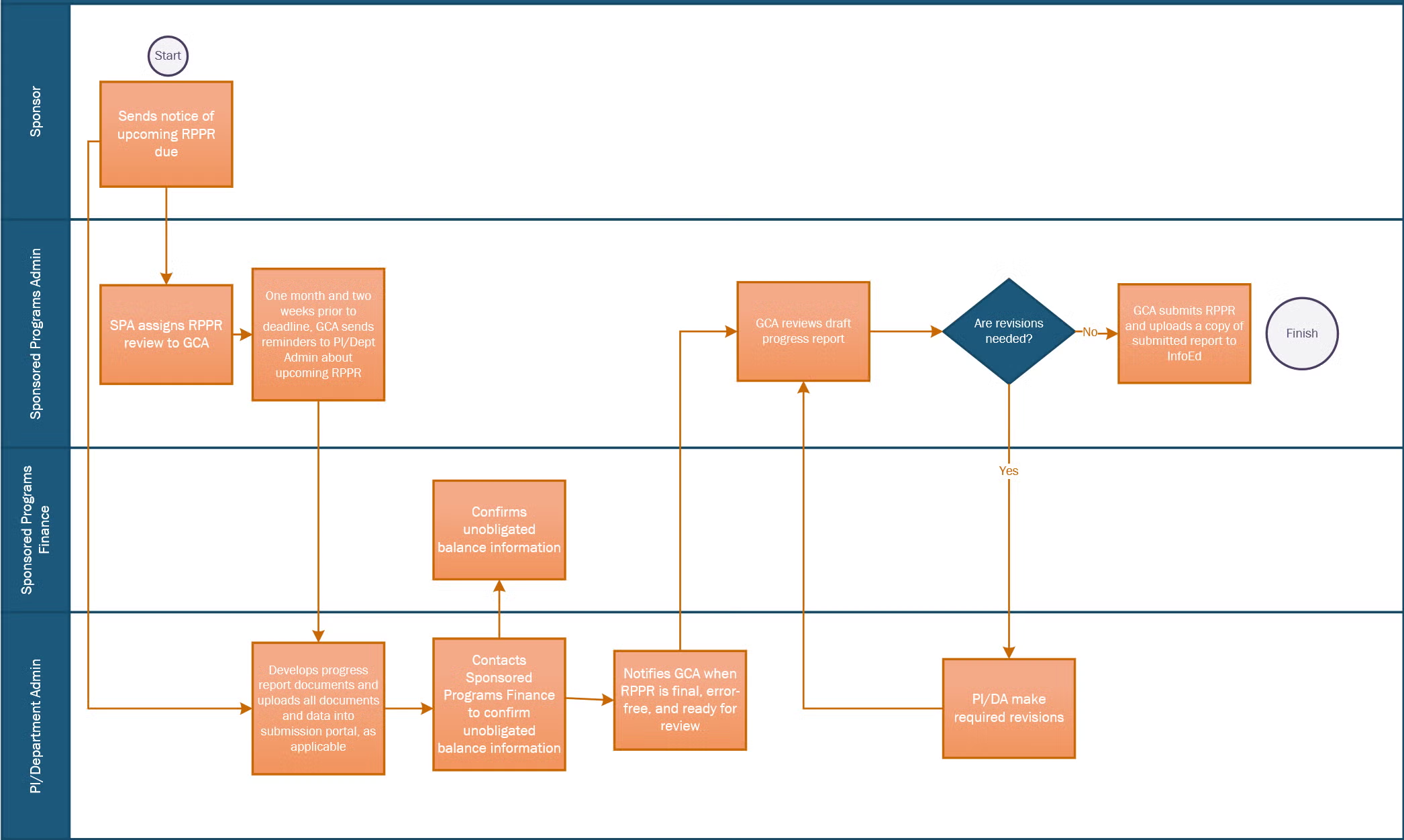
The Research Performance Progress Reports (RPPR) serves as the official progress report document for grantees to submit progress updates to NIH. It documents the accomplishments of grantees and compliance with the terms of the award. This page outlines important requirements and procedures to ensure a complete and compliant RPPR, and how to navigate the BMC submission process.
Communications
- Two months before the deadline, eRA Commons sends an automated reminder to the PD/PI about the upcoming RPPR deadline. This is the ideal time to begin preparing the RPPR. Note that only the PI or their official delegate can initiate an RPPR in eRA Commons.
- Your SPA GCA will also send courtesy reminders one month and two weeks before the deadline.
Special Instructions on Required Elements
- Consult the RPPR Guide for current instructions on developing your RPPR: https://grants.nih.gov/grants/rppr/rppr_instruction_guide.pdf
- Section A: List the SPA Associate Director or SPA Manager as Administrative Official. Your department’s assigned SPA GCA should be listed as the Signing Official.
- Section C: Ensure publications comply with the NIH Public Access Policy before submitting to SPA.
- Section D: If Other Support documents are needed, please confirm they are in the current format and include all four required sections (Active, Pending, In Kind, Overlap). Effort can not exceed 12 calendar months; please address overlap if it does. All Other Support documents must be electronically signed (Docusign, Adobe sign, etc.). Instructions, templates, and FAQs can be found here: https://grants.nih.gov/grants/forms/othersupport.htm.
- Section G.4/ASSIST Human Subjects Section: For awards including clinical trials, updates to clinicaltrials.gov must be submitted at least two weeks before the RPPR deadline. Contact the BMC/BU PRS Administrator for assistance.
- Section G.10: Confirm the unobligated balance projection with Sponsored Programs Finance before sending the report to SPA. Forward the confirmation email to your SPA GCA.
Submission Process
- Check your RPPR for errors. Correct all errors prior to routing the RPPR to SPA.
- Submit your final, error-free report to SPA at least five business days before the deadline. The department will route the RPPR in eRA Commons to the SPA GCA assigned to your department when it is ready for review.
- The SPA GCA will review the RPPR. If revisions are needed, the RPPR will be routed back to you with notes about the required changes. Once finalized and approved, the SPA GCA will submit the RPPR to the NIH in eRA Commons as the Signing Official.
For additional information, see the National Institutes of Health Grants Policy Statement on reporting.
Non-NIH Progress Reports
For progress reports required by non-NIH sponsors, review your agreement and/or sponsor policies to understand reporting requirements. Contact your SPA GCA with any questions you have about submitting progress reports to non-NIH sponsors.
Resources
 ht
ht 
 English
English Français
Français Deutsch
Deutsch Italiano
Italiano Español
Español Tiếng Việt
Tiếng Việt